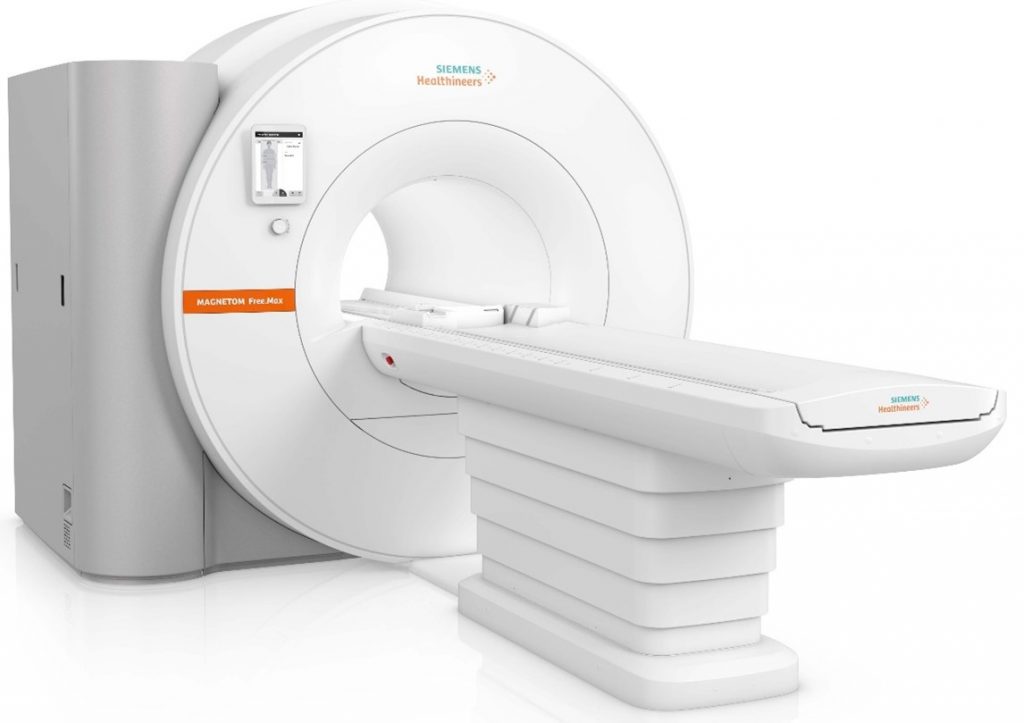
The U.S. Food & Drug Administration has given clearance to a new, smaller magnetic resonance imaging scanner from Siemens Healthineers that employs the company’s technology to greatly reduce the amount of helium needed for cooling the magnet system. With a magnetic field strength of 0.55 Tesla, it opens the door to more widespread use of MRI systems in healthcare.
At less than 3.5 tons and less than 80 inches high, the MAGNETOM Free.Max is the most lightweight, compact whole-body scanner ever offered by the company. Its reduced size permits installation with minimal structural modifications. And where MR scanners typically require several hundred liters of helium and a quench pipe for cooling purposes, the new magnet of the MAGNETOM Free.Max uses less than 1 liter of helium, reducing lifecycle and infrastructure costs, enabling it to be installed in areas where an MR scanner could not be housed previously. The first and only 80 cm wide-bore system available, the MAGNETOM Free.Max also facilitates MR scanning for extremely obese and claustrophobic patients.
“Siemens Healthineers is proud to offer the MAGNETOM Free.Max, which brings MR to new clinical fields with innovative digital technology, new siting features, and image quality that was once realized only at higher field strengths,” said Jane Kilkenny, vice president of magnetic resonance at Siemens Healthineers North America. “The scanner’s comparatively low weight and size can open the door to MR utilization in orthopedic centers, emergency rooms, outpatient centers, and even intensive care units.”
MAGNETOM Free.Max systems from Siemens are equipped with DryCool technology, an innovative sealed-for-life magnet design that operates on only 0.7 liters of liquid helium. It eliminates the need for additional helium infrastructure, including the cumbersome installation of a quench pipe. For conventional MRIs that operate on hundreds of liters of helium, a quench pipe is needed to safely expel the helium out of the building in emergencies where the magnet needs to be shut off instantly.
For conventional MRI scanners, notes Siemens, a quench typically has a significant financial impact caused by scanner downtime. This is linked to the time required for the helium refill and reimbursement shortfalls caused by the rescheduling of patients or if patients simply choose other MRI providers in the meantime. With DryCool, however, the magnet can quickly and automatically be ramped up again after the emergency has been resolved, as the small amount of liquid helium always remains in the magnet. The entire process takes less than a day without any further costs.
Headquartered in Erlangen, Germany, Siemens Healthineers operates in the U.S. as Siemens Medical Solutions USA, based in Malvern, Pennsylvania. For more info, see www.siemens-healthineers.com.



