Bolstered with $58 million in cash and piling up treatment authorizations, medical device maker Brainsway is poised to grow the market for magnetic-based neurostimulation to treat a host of brain disorders such as obsessive-compulsion and depression as well as smoking addiction. The authorizations are coming from medical certification authorities and — not be overlooked — insurance carriers.
Among a string of recent achievements, in June the company received another authorization for insurance coverage of OCD treatment using its Deep TMS transcranial magnetic stimulation system, the latest one coming from Health Care Service Corporation. An independent licensee of Blue Cross Blue Shield Association and the fourth largest health insurance company in America, HCSC covers approximately 17 million members.

“Coming on the heels of two other major recent OCD coverage announcements, this latest milestone is indicative of the strong reimbursement momentum we are experiencing in support of our Deep TMS treatment for OCD. We will continue our work to drive further positive coverage developments in this important indication from additional BCBS systems and other major health plans,” said Christopher von Jako, CEO.
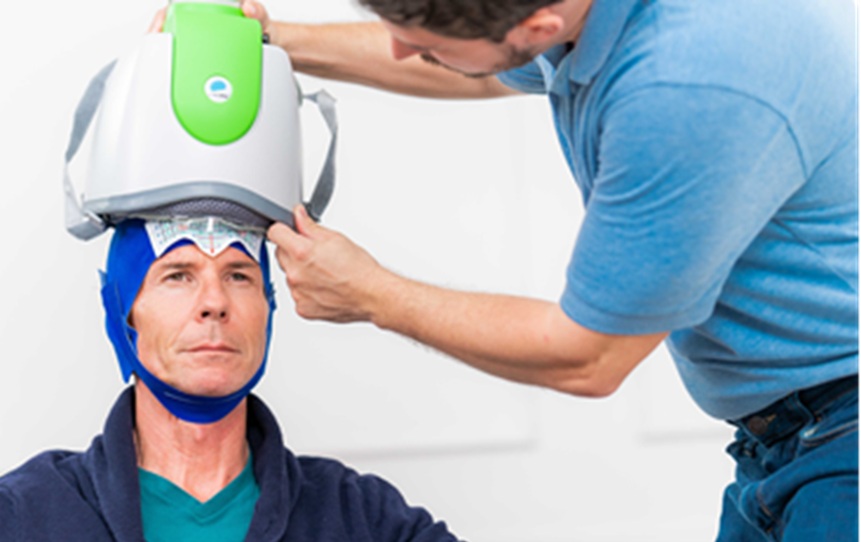
Deep TMS, has been shown to safely and effectively alleviate the symptoms of obsessive-compulsive disorder, particularly those patients who have not achieved sufficient improvement from traditional OCD treatment options. The treatment utilizes a magnetic field emitted by BrainsWay’s patented H7 coil to directly reach broader and deeper brain regions than its predecessors, regulating the neural activity of brain structures associated with OCD, specifically the anterior cingulate cortex and medial prefrontal cortex. A peer-reviewed multicenter clinical study found Deep TMS to be a highly effective OCD treatment, with more than one in three treatment-resistant OCD patients achieving response, greatly improving their quality of life. As a non-invasive procedure, Deep TMS is a well-tolerated treatment that does not cause any adverse or long-lasting side effects. It does not require a significant recovery period, and the 18-min treatment can easily be integrated into a patient’s day-to-day schedule.
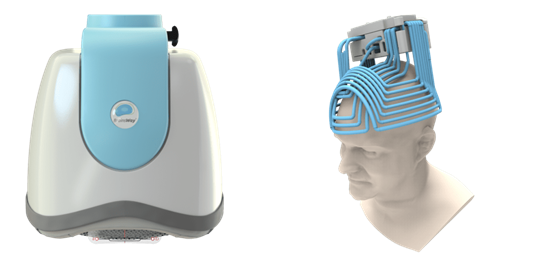
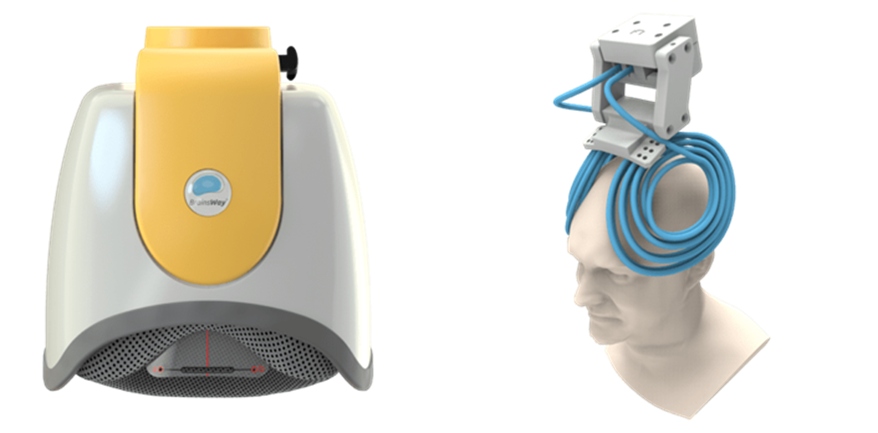
The company’s Deep TMS flagship technology is based on the H-Coil which features a novel patented structure designed to maximize electrical stimulation of neurons in deep brain regions. The coil elements are tangential to the head and close to target brain regions with a flexible base that can be suited to head shape. The system is designed for convergence of numerous electric pulses from various directions with the coil elements parallel to target bundles and location of return paths of electrical impulses remote from the target area.

To date, more than 100,000 patients have been treated globally with Deep TMS. The technology received FDA clearance for the treatment of major depressive disorder in 2013 and for the treatment of OCD in 2018. More recently, it was FDA-cleared as an aid for short-term smoking cessation.
In April, Brainsway was granted FDA clearance for its Theta Burst three-minute protocol utilizing its Deep TMS system for the treatment of major depressive disorder. “Adding our three-minute treatment protocol to the list of growing solutions available to our provider partners expands the platform nature of this lifechanging technology,” said Hadar Levy, senior vice president and general manager. “Many patients and providers can benefit from significantly shorter treatment sessions, and our Theta Burst protocol can provide these patients with another option to manage their treatment resistant depression.”
Now, armed with fresh cash and rising revenues, Brainsway looks poised to grow its business further. In May, the company reported it was holding $58.5 million cash, primarily the result of a public offering in March that brought in $45 million. Revenues climbed to $6 million during the first three months of this year, climbing 47% over the same period in 2020. By the end of April, it had already sent out its first ten devices for smoking addiction to practices across the U.S., which use its H4 coil and cushioned helmet.
Founded in 2003, Brainsway operates from offices in Cresskill, NJ and Jerusalem, Israel. For more info, see www.brainsway.com.