Cochlear Limited, a global developer of implantable hearing solutions, recently received U.S. Food and Drug Administration (FDA) clearance of its new Cochlear Osia 2 System that employs innovative sound magnets and magnetic attachment. The Osia System is the world’s first active osseointegrated steady-state implant (OSI), a new category of bone conduction hearing solutions that uses digital piezoelectric stimulation to bypass damaged areas of the natural hearing system to send sound vibrations directly to the inner ear (cochlea).
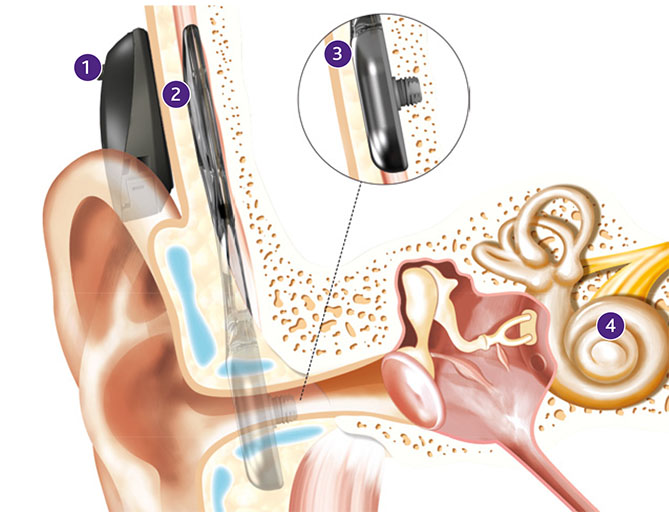
“The Osia 2 Sound Processor sits off-the-ear on the patient’s head and is retained through a magnet in the sound processor attracted to a magnet in the implant underneath the skin,” said Anna Anderstroem, Cochlear Americas’ lead bone conduction group leader, who provided details to Magnetics Magazine about the magnetics technology involved.

“Osia 2 Sound Processor magnets are available in four different strengths (1 – 4), covering skin thicknesses up to 9mm. The sound processor magnet material is neodymium. The polarity of the magnets in the sound processor can be reversed by placing the magnets upside down in the sound processor. The magnet assembly in the OSI200 Implant ensures that the sound processor is firmly attached to the patient’s head and correctly aligned with the implant RF receiver coil. The magnet assembly in the OSI200 Implant has a laser engraved ring that indicates the south magnetic pole of the magnet. Both the shells and engraving are smooth to minimize the risk of bacterial growth.”
“A pocket in the silicone overmold of the OSI200 Implant has been designed to hold the magnet assembly securely in place and prevent it from being dislodged by a severe impact, physical manipulation or external magnetic fields such as the sound processor magnet,” said Anderstroem. “To achieve this, the silicone pocket is slightly smaller than the magnet in order to apply compressive force to cinch it in place. An opening in the silicone pocket allows the removal and replacement of the magnet should this be required after implantation, as may be needed if the recipient were to undergo a 3T (or higher) magnetic resonance imaging procedure.”
In March, the U.S. Food and Drug Administration approved to lower the age of cochlear implantation from 12 months to 9 months for children with bilateral, profound sensorineural hearing loss. This approval ensures children born deaf have earlier access to a cochlear implant which can provide them with the hearing capabilities to develop speech and language at a trajectory like their hearing peers.

According to the American Academy of Pediatrics, an estimated three in 1,000 infants are born in the U.S. each year with moderate, severe or profound hearing loss. Additionally, hearing loss is the most common congenital condition in the U.S. Research shows that children with a severe-to-profound hearing loss who receive cochlear implants early achieve better speech recognition than children who continue to use hearing aids, underscoring the importance to not delay access to cochlear implants because doing so can have lingering developmental consequences.

Headquartered in the suburbs of Sydney, Australia, Cochlear is a global leader in implantable hearing solutions. The company has a workforce of more than 4,000 people worldwide and invests more than $128 million each year in research and development. Products include cochlear implants, bone conduction implants and acoustic implants, which healthcare professionals use to treat a range of moderate to profound types of hearing loss. Cochlear Americas is based in Centennial, CO near Denver.
For more info, see www.cochlear.com.