
Scientists at Lawrence Berkeley National Laboratory have made a new material that is both liquid and magnetic, opening the door to a new area of science and potential product development in magnetic soft matter. Basically, they have managed to print magnetic liquid droplets. Their findings could lead to a revolutionary class of printable liquid devices for a variety of applications from artificial cells that deliver targeted cancer therapies to flexible liquid robots that can change their shape to adapt to their surroundings.
“We’ve made a new material that is both liquid and magnetic. No one has ever observed this before,” said Tom Russell, a visiting faculty scientist at Berkeley Lab in California and professor of polymer science and engineering at the University of Massachusetts, Amherst, who led the study. “This opens the door to a new area of science in magnetic soft matter.”
For the past seven years, Russell, who leads a program called Adaptive Interfacial Assemblies Towards Structuring Liquids in the lab’s Materials Sciences Division, has focused on developing a new class of materials – 3D-printable all-liquid structures. Their findings were published July 19 in the journal Science.
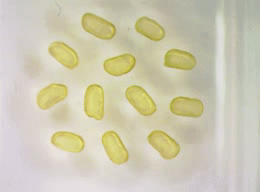
The image above shows an array of 1 millimeter magnetic droplets. Fluorescent green droplets are paramagnetic without any jammed nanoparticles at the liquid interface, red are paramagnetic with nonmagnetic nanoparticles jammed at the interface, brown droplets are ferromagnetic with magnetic nanoparticles jammed at the interface.
Russell and Xubo Liu, the study’s lead author, came up with the idea of forming liquid structures from ferrofluids which are solutions of iron-oxide particles that become strongly magnetic in the presence of another magnet. “We wondered, ‘If a ferrofluid can become temporarily magnetic, what could we do to make it permanently magnetic, and behave like a solid magnet but still look and feel like a liquid?’” said Russell.
To find out, they used a 3D-printing technique they had developed with former postdoctoral researcher Joe Forth at the lab to print 1 millimeter droplets from a ferrofluid solution containing iron-oxide nanoparticles just 20 nanometers in diameter — the average size of an antibody protein.
Using surface chemistry and sophisticated atomic force microscopy techniques, staff scientists Paul Ashby and Brett Helms of the lab’s Molecular Foundry revealed that the nanoparticles formed a solid-like shell at the interface between the two liquids through a phenomenon called “interfacial jamming.” This causes the nanoparticles to crowd at the droplet’s surface, “like the walls coming together in a small room jampacked with people,” said Russell.
To make them magnetic, the scientists placed the droplets by a magnetic coil in solution. As expected, the magnetic coil pulled the iron-oxide nanoparticles toward it.
But when they removed the magnetic coil, something quite unexpected happened.
Permanently magnetized iron-oxide nanoparticles gravitate toward each other in perfect unison.
Like synchronized swimmers, the droplets gravitated toward each other in perfect unison, forming an elegant swirl “like little dancing droplets,” said Liu, who is a graduate student researcher at the lab and a doctoral student at Beijing University of Chemical Technology and is credited for the accompanying photos.
Somehow, these droplets had become permanently magnetic. “We almost couldn’t believe it,” said Russell. “Before our study, people always assumed that permanent magnets could only be made from solids.”
Measure by measure, they confirmed they indeed had a magnet. All magnets, no matter how big or small, have a north pole and a south pole. Opposite poles are attracted to each other, while the same poles repel each other.
Through magnetometry measurements, the scientists found that when they placed a magnetic field by a droplet, all of the nanoparticles’ north-south poles, from the 70 billion iron-oxide nanoparticles floating around in the droplet to the 1 billion nanoparticles on the droplet’s surface, responded in unison, just like a solid magnet.
Key to this finding were the iron-oxide nanoparticles jamming tightly together at the droplet’s surface. With just 8 nanometers between each of the billion nanoparticles, together they created a solid surface around each liquid droplet.
Somehow, when the jammed nanoparticles on the surface are magnetized, they transfer this magnetic orientation to the particles swimming around in the core, and the entire droplet becomes permanently magnetic – just like a solid, Russell and Liu explained.
The researchers also found that the droplet’s magnetic properties were preserved even if they divided a droplet into smaller, thinner droplets about the size of a human hair, added Russell.
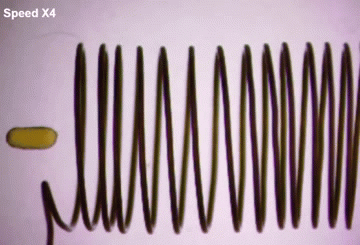
To make the iron-oxide nanoparticles permanently magnetic, the scientists placed the droplets by a magnetic coil in solution. As expected, the magnetic coil pulled the iron-oxide nanoparticles toward it.
Among the many amazing qualities of the droplets, what stands out even more, Russell noted, is that they change shape to adapt to their surroundings. They morph from a sphere to a cylinder to a pancake, or a tube as thin as a strand of hair, or even to the shape of an octopus – all without losing their magnetic properties.
They can also be tuned to switch between a magnetic mode and a nonmagnetic mode. And when their magnetic mode is switched on, their movements can be remotely controlled as directed by an external magnet, Russell added.
Liu and Russell plan to continue research at Berkeley Lab and other national labs to develop even more complex 3D-printed magnetic liquid structures, such as a liquid-printed artificial cell, or miniature robotics that move like a tiny propeller for noninvasive yet targeted delivery of drug therapies to diseased cells.
“What began as a curious observation ended up opening a new area of science,” said Liu. “It’s something all young researchers dream of, and I was lucky to have the chance to work with a great group of scientists supported by Berkeley Lab’s world-class user facilities to make it a reality,” said Liu.
Also contributing to the study were researchers from UC Santa Cruz, UC Berkeley, the WPI–Advanced Institute for Materials Research (WPI-AIMR) at Tohoku University, and Beijing University of Chemical Technology.
The full article is available at https://science.sciencemag.org/content/365/6450/264.



