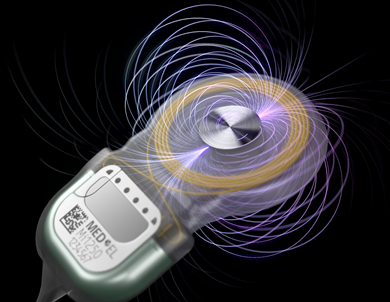
MED-EL, a maker of medical hearing devices based in Austria, has beefed up the strength of the diametric magnet in its cochlear implants while still retaining their ability to pass through magnetic resonance imaging systems. The company is also making breakthroughs with the development of totally implantable magnetic cochlear implants.
In 2014, MED-EL rocked the hearing implant industry with the launch of Synchrony, the world’s first cochlear implant featuring a patented diametric magnet approved for 3.0T MRI’s without requiring magnet removal. Now the company is taking its innovative magnet design to the next level by releasing an all-new diametric magnet with S-Vector technology. Approved by the U.S. Food and Drug Administration (FDA), the S-Vector magnet is a feature of Synchrony 2 cochlear implants that began shipping in March.
“Over the past 25 years, we have continuously developed cutting-edge magnet designs to tackle the issue of MRI compatibility and hearing implants for our recipients,” said Raymond Gamble, president & CEO of MED-EL North America. “We’re thrilled to announce that we’ve further advanced our magnet design and have now made the second generation available to the U.S. market.”
“A stronger implant magnet results in a stronger holding force, providing outstanding flexibility in finding the ideal fit with the user‘s audio processor,” said Martin Zimmerling, head of implant development at MED-EL. “The S-Vector provides more holding force and provides a great option for adapting to the user’s individual needs. This gives our recipients peace of mind knowing their audio processors will stay securely in place.”
With a 25% increase in magnetic strength, the new device is ideal for active recipients with off-the-ear single-unit audio processors, the company says. Despite increased magnetic strength, there are no changes to existing MRI scanning instructions. The S-Vector magnet has the same small MRI artifact as the first-generation magnet. In a diametric magnet such as MED-EL employs, the neodymium disc is magnetized across the diameter. When the disc is sitting flat on a surface, the north and south poles are located on the curved sides, at opposite side.
First surgeries in Europe with a totally implantable cochlear implant
Last fall the company reached another milestone when, on September 24, surgery took place for the first person in Europe to receive a totally implantable cochlear implant as part of a clinical feasibility study at the University of Liege medical center in Belgium. The device is expected to give users great hearing with even more comfort, though it is expected to take several years before receiving market approval.

“Our cochlear implants have helped hundreds of thousands of people around the world to hear and have improved their quality of life. Many users have expressed the wish for a CI that works without an external component on or off the ear, is invisible and operates even when users are asleep “, says Dr. Ingeborg Hochmair, founder and CEO of MED-EL.
Cochlear implant systems, which bypass the non-functioning part of the ear and stimulate the hearing nerve electrically, have become a standard treatment for individuals with severe to profound hearing loss. Traditionally, they consist of an internal implant which is surgically placed underneath the skin, and an audio processor which is worn externally, behind or off the ear. The audio processor contains the microphone required to pick up sound as well as the power supply.
Totally implantable cochlear implants (TICI), however, are a newer modality. They contain all the internal and external components of a cochlear implant system in one device placed underneath the skin, including the audio processor, microphone and power supply.
“We tested the implant after surgery and are thrilled that everything is working as expected. Modern cochlear implant technology has been evolving at an impressive pace, delivering outstanding hearing results. The TICI is a milestone within the field of cochlear implantation. It has been a wish from the early days of cochlear implantation to be able to integrate all components within an internal device,” said Prof. Dr. Philippe Lefebvre, head of the ENT Department of the CHU of Liege and Professor at the University of Liege, who performed the surgery in September. Several more surgeries were to follow in the study.

Headquartered in Innsbruck, MED-EL has grown to more than 2,200 employees since hiring its first in 1990. The company makes a wide range of devices to treat all types of hearing loss including cochlear implant systems, a combined Electric Acoustic Stimulation hearing implant system, as well as surgical and non-surgical bone conduction devices. For more info, see www.medel.com.



