
Endomag, the British company that is now a prized holding of Massachusetts-based medical device maker Hologic, has achieved EU Medical Device Regulation certification for its Magseed marker. Confirming it meets the most rigorous European standards for medical devices, the door-opening approval was conferred in October 2025. Meanwhile, its Magtrace super paramagnetic iron oxide beads product continues to gain important recognition through research studies, further raising the profile of the company’s integrated product line for detecting and treating breast cancer.
“Achieving EU MDR certification for the Magseed marker is testament to the importance we place on product quality, rigorous evidence of clinical effectiveness and engineering expertise. Navigating one of the toughest regulatory processes means clinicians can have even greater assurance in the safety and reliability of the technology they depend on to support their patients every day,” said Mehryar Behizad, Regulatory Director.
More than 500,000 patients already have been treated with the Magseed marker, establishing a wire-free revolution in breast cancer diagnosis and treatment. For details on Endomag’s progress and its technology, see previous reports in Magnetics Magazine.
The future is magnetic
“The future is magnetic”, declares Hologic, which acquired Endomag and its integrated magnetic-based product line in 2024 for $310 million. Whether it’s a simple and reliable seed which magnetizes to guide surgeons closer to the tumor site, or the tracer designed to identify the most suspicious lymph nodes, magnetism provides a proven flexible alternative to traditional radioactive techniques, the company notes. Hologic is a global leader in women’s health that develops innovative medical technologies to detect, diagnose and treat health conditions.
Magtrace efficacy mounts
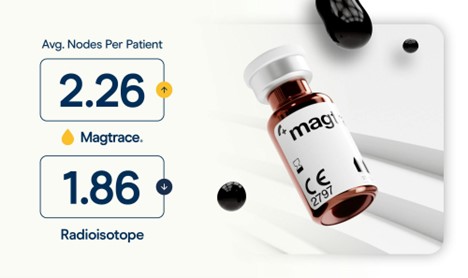
Topping a mounting body of research, a recent meta-analysis has added to the growing body of evidence supporting the Magtrace lymphatic tracer for use in sentinel lymph node biopsy after neoadjuvant chemotherapy. Boland et al (2025) found that the Magtrace lymphatic tracer performs on par with radioisotope and blue dye for detection accuracy, with the added benefit of identifying more nodes and offering greater procedural flexibility. The paper assessed outcomes from five studies involving 374 breast cancer patients and compared Magtrace to the traditional radioactive standard.
For more info, see www.endomag.com and www.hologic.com.



