
Advancements in transcranial magnetic stimulation, otherwise known as TMS, are yielding new diagnostic and treatment solutions for a growing roster of neural conditions. The new TMS products are bringing expanding business opportunity to medical device companies as they pioneer promising new therapies for patients.
This article looks at the recent progress of several innovative device makers notably eNeura and its products for treating migraine (pictured above), MagStim systems for combatting depression, and Neuroelectric’s StarSim system for treating epilepsy. These represent just a few of the developments taking place as TMS is now being studied in treatments for brain-based conditions ranging from ringing in the ears to Alzheimer’s disease, from memory disorders to depression.
A common thread is that TMS is steadily moving into mainstream treatment. These companies and others along with neuromedicine specialists are meeting with success in a mounting number of agency approvals based upon progress in demonstrating treatment effectiveness and safety, as well as acceptance by the medical community and patients.
How TMS works
In TMS, current is passed through specially shaped electrical coils to generate magnetic fields of particular strength and lasting for precise lengths of time. Through the process of induction, these magnetic fields can pass painlessly through bone and tissue, stimulating complementary electrical activity within the brain that can be visualized by magnetic resonance imaging systems.
In its early years, TMS was used to study brain function, allowing researchers to explore the ways that nerves conduct electricity, what parts of the brain were involved with speech and emotion, and the impact of stroke on the brain. Continuing research has led to investigation of its use in a wide range of neuromodulation therapies. Because the brain works through the conduction of electrical impulses, the research and development has focused on the intentional introduction of mild electrical fields to specific parts of the brain seeking to modulate brain activity in a manner that yields important health benefits.
eNeura sMTS for migraine therapy
In February, eNeura, Inc., a medical technology company based in Baltimore, obtained clearance from the U.S. Food and Drug Administration to expand the application of its portable, patient-controlled sMTS device for acute and preventive treatment of migraine to include children 12 years of age and older. The company received clearance for acute and preventive treatment of migraine in adults in 2017. With the new clearance, its device became the only migraine product in the United States available for the acute and prophylactic treatment of migraine headache in adults and children. More than four million children, 12 years and age and older suffer from migraine headache in the US and more than 36 million people in the US suffer from migraine headache, says eNeura.
Its sTMS is a non-invasive, prescription-only device that utilizes single-pulse TMS (sTMS) to induce a mild electric current that modulates nerve cells in the brain and which is believed to interrupt the brain hyperactivity associated with migraine. Patients place the device at the back of the head, and with the simple push of a button, deliver a focused magnetic pulse to treat a migraine attack and or to prevent the onset of future migraine attacks. Clinical trials have shown sTMS is as effective as migraine medications but without the risks or unwanted side effects.
“The clearance of our sTMS product for both acute treatment and prevention of migraine in children as young as 12 years old is a breakthrough for migraine headache patients in the U.S.,” commented Dr. David K. Rosen, president and CEO of eNeura. “Until now, children have had very few safe and effective options for the treatment and prevention of migraine. Typically, migraine patients, including children, have had to use combinations of pharmaceutical products, each with potentially unpleasant and often disabling side-effects, to prevent headache and treat acute headache attacks. sTMS is now labeled to address the entire spectrum of migraine with an easy-to-use device, that in multiple clinical studies, has proven to be safe and effective.”
MagStim TMS system for treatment of depression
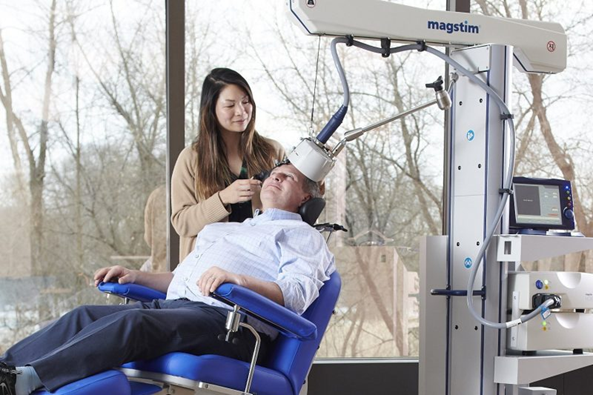
MagStim, a pioneer in developing TMS for treatment of depression for more than 30 years, has recently received important clearances for its equipment to be used more broadly. Its stimulators and coils are designed and manufactured at its headquarters in West Wales, UK. The company employs about 100 people, the majority of which work there and at its other main office in Eden Prairie, MN.
In depressed patients, electrical activity in certain areas of the brain is reduced. Its Horizon system uses a focused electromagnetic coil to rapidly pulse a magnetic field to the targeted area of the brain. This induces a small electrical current which stimulates the targeted brain cells into activity, gradually increasing brain activity back to a normal level. With a range of treatment protocols available in one system, Magstim gives practitioners the flexibility to tailor their treatments for patients without the need to change equipment.
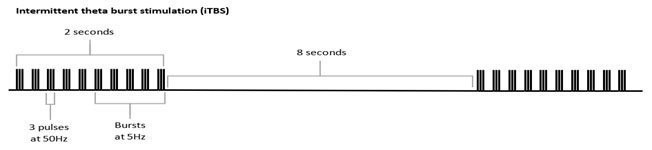
In March, Magstim received FDA clearance to include intermittent Theta Burst Stimulation (iTBS) as a treatment for major depressive disorder with its Horizon systems.
This clearance allows Magstim to market the 3-minute iTBS protocol in the US now. It is already available in the UK and EU. Clinicians have options to deliver treatments with the standard repetitive Transcranial Magnetic Stimulation (rTMS), which takes 37.5 minutes, accelerated TMS at 19 minutes, and iTBS, which takes just three minutes per treatment. Horizon was designed with a proprietary energy recovery system to ensures consistency throughout the treatment with no pulse amplitude decay no matter the protocol, a feature which the company says is unique. The system is equipped with a versatile E-z Cool Coil which has a longer pulse width that is optimized for patient comfort and has been shown to be more tolerable for patients.
“I am pleased that we can now market the full capabilities of the Horizon Performance platform,” commented Lothar Krinke, MagStim Group CEO. “We want to provide innovative systems that allow clinicians and researchers to customize and personalize treatment for patients, and this clearance is another step in that direction.”

Magstim Group CEO Lothar Krinke
In June, Magstim made another step forward when it launched what it says is the first TMS navigation system specifically developed for the clinical market. Used with its Horizon system, the company’s new StimGuide provides precise and consistent coil positioning in the treatment of major depressive disorder. The 3D navigation system aligns four distinct parameters to guide clinicians to a precise target area for treatment, reducing any potential variability in treatment and improving confidence and consistency in the delivery of the TMS therapy.
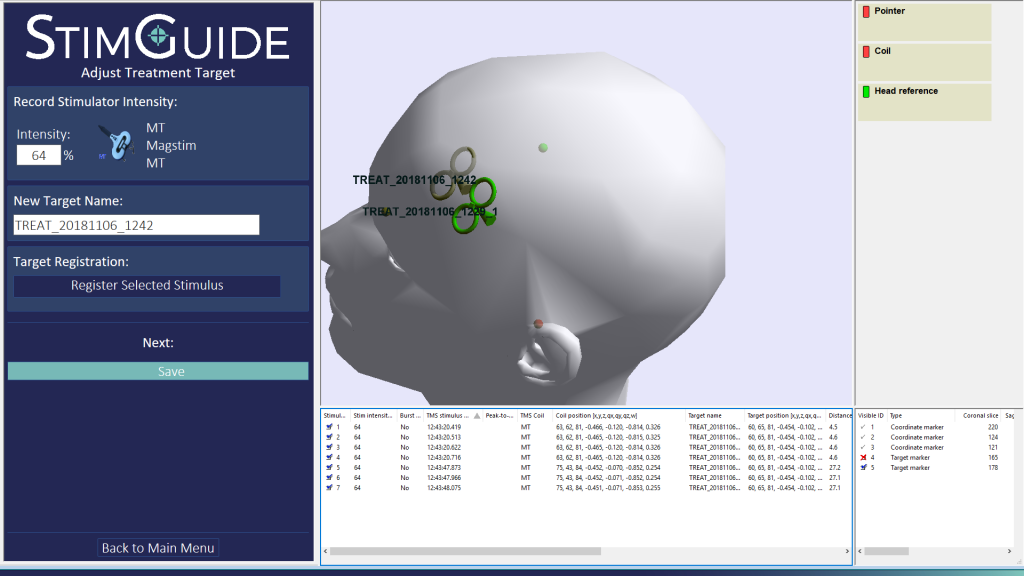
StimGuide was developed to provide a solution for clinical centers that do not have access or the resources to provide pre-treatment MRIs for patients. It offers the benefits of 3D navigation without the expense and complexity associated with more complicated navigation technology. It provides an option to a one-dimensional contact sensor enabling a solution that can leapfrog a cap and measuring method or contact sensor technology and provides precise and repeatable coil positioning, said Krinke

“I am convinced that StimGuide will improve patient care because it ensures reliable placement over the intended treatment target providing confidence to experienced and new TMS treaters. It accurately measures the distance of the coil from the intended target as well as rotation and pitch ensuring every pulse train is delivered in the optimal coil position.”

MagStim’s Rapid system for research
Other products from MagStim include a variety of coils, and its Rapid family of research equipment which is compatible with the full range of Magstim coils and has the ability to connect an EMG module suitable for high-frequency repetitive protocols for both cortical and peripheral stimulation.
Neuroelectrics Starsim device for combatting epilepsy

Neuroelectrics’ Starstim device for epilepsy treatment
Neuroelectrics Corporation, manufacturer of a research-grade TMS system for treating epilepsy, is making significant strides in advancing its Starsim product to being authorized for clinical treatment of patients. At this year’s American Epilepsy Society Annual Meeting in New Orleans, it reported important results from a clinical trial treating patients with drug resistant epilepsy with the device which uses mild electric currents applied on the scalp to calm abnormal activity of the brain.
Of the 17 patients that completed the study, treatment with its device resulted in a reduction in seizure frequency of at least 40% from baseline in 75% of the patients, measured eight weeks after treatment. Also, no device-related adverse events were reported during the study.
Neuroelectrics sponsored the FDA-approved investigational device study at Boston Children’s Hospital. All patients had not responded to at least two anti-epileptic medications, and for many the next step would be brain surgery to resect the region of the brain where the seizures originate. The treatment protocol used 20 minutes of daily stimulation applied for 10 days times over two weeks, followed by an eight-week monitoring period to measure seizure frequency.
According to Alexander Rotenberg, MD, PhD, associate professor of neurology at the hospital and at Harvard Medical School, who was co-principal investigator of the study, “We and our patients are delighted to have a non-invasive and non-pharmacologic option for those whose seizures have not been controlled by drugs or by surgery. Our patients and families have seen clear improvements in well-being and quality of life.”
The system potentially could have a broad impact since 60 million patients, approximately 1% of the population worldwide, live with epilepsy and it is estimated that nearly a third of them do not have their seizures well controlled by medications. The therapy has the potential to be used for both patients who are not candidates for epilepsy surgery, and patients who have undergone epilepsy surgery but continue to have seizures.

The company’s technology develops a personalized stimulation protocol to target the specific areas of the patient’s brain where the seizures originate, as identified by the treating neurologist. Then the wireless device, housed in a neoprene headcap, is worn for 20 minutes per day with no penetration of the brain or skin. It’s 85G model, for example, provides 39 positions where electrodes can be easily inserted.

Ana Maiques, CEO of Neuroelectrics
Ana Maiques, Neuroelectrics CEO, said: “We are pleased that this clinical study has demonstrated the safety and efficacy of Starstim in treating epilepsy. We are proud of having conducted the first FDA-sanctioned clinical trial in transcranial current stimulation in any indication, positioning Neuroelectrics as the leader in developing an option for patients that employs this powerful therapeutic brain stimulation technology.” The company operates from its headquarters in Barcelona and an office in Boston.
For more info, see www.eneura.com; www.magstim.com; and www.neuroelectrics.com.



