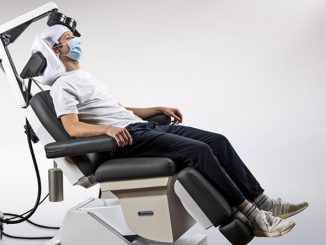
BrainsWay Ltd., a global leader in advanced noninvasive treatment of brain disorders, today announced the distribution of its first 10 Deep Transcranial Magnetic Stimulation (Deep TMS) devices for smoking addiction to practices across the United States. The recently FDA-cleared aid for short-term smoking cessation offers a noninvasive treatment that administers electromagnetic pulses using BrainsWay’s patented H4-coil through a cushioned helmet, stimulating neurons in brain structures associated with addiction.
“Tobacco smoking is one of the leading causes of preventable disease and death throughout the world,” said Christopher von Jako, Ph.D., President and Chief Executive Officer of BrainsWay. “We have seen lives drastically changed after undergoing Deep TMS treatment and are eager to bring this same hope to those who desire to quit smoking. Research shows that there is often comorbidity between tobacco use and mental disorders, and the COVID-19 pandemic has exacerbated both. Many patients have given up trying to quit, but now, with a noninvasive option like Deep TMS, we are hopeful they are able to take that step.”
Deep TMS is the first FDA-cleared noninvasive treatment in the addiction space for any TMS device, and currently serves as the only TMS platform technology with clinical outcome data for multiple brain disorders. In 2019, a double-blind, sham-controlled, multicenter randomized controlled trial of 262 patients found Deep TMS to be an effective treatment, significantly improving the continuous quit rate, as well as reducing craving and the average number of cigarettes smoked per week. Participants in the study were highly addicted to smoking with a history of smoking an average of over 26 years and multiple failed attempts to quit.
Using BrainsWay’s patented H4 Coil, the treatment generates electromagnetic pulses that stimulate neurons in brain structures associated with addiction – specifically the bilateral insula and prefrontal cortex. BrainsWay Deep TMS is proven to reach wider and deeper brain regions than traditional TMS devices.
A double-blind, sham-controlled, multicenter randomized controlled trial of 262 patients found Deep TMS to be an effective treatment, significantly improving the continuous quit rate, reducing craving and the average number of cigarettes smoked per week. Participants in the study were highly addicted to smoking, with a history of smoking an average of over 26 years and multiple failed attempts to quit.
Deep TMS is a well-tolerated, noninvasive treatment with no systemic side effects. It does not require a significant recovery period, and the 18-min treatment can easily be integrated into each patient’s day-to-day schedule.
For more info visit www.brainsway.com