Physicists at JILA have created a crystal-like arrangement of ultracold gas molecules that can swap quantum “spin” properties with nearby and distant partners. The novel structure might be used to simulate or even invent new materials that derive exotic properties from quantum spin behavior, for electronics or other practical applications.

Described in a Nature paper* posted online on Sept. 18, 2013, the JILA experiment is the first to record ultracold gas molecules exchanging spins at a distance, a behavior that may be similar to that of intriguing solids such as “frustrated” magnets with competing internal forces, or high-temperature superconductors, which transmit electricity without resistance. The new results build on the same JILA team’s prior creation of the first molecular quantum gases and demonstrations of ultracold chemistry.**
JILA is a joint institute of the National Institute of Standards and Technology (NIST) and the University of Colorado Boulder.
“One of the main thrusts for our cold molecules research was to realize this interaction, so this is a major breakthrough,” NIST/JILA Fellow Jun Ye said. “We can now explore very exotic new phases of quantum systems.” NIST/JILA Fellow Deborah Jin points out that “these interactions are advantageous for creating models of quantum magnetism because they do not require the molecules to move around” the crystal structure.
The new JILA crystal has advantages over other experimental quantum simulators, which typically use atoms. Molecules, made of two or more atoms, have a broader range of properties, and thus, might be used to simulate more complex materials. Jin and Ye are especially interested in using the structure to create new materials not found in nature. An example might be topological insulators, a hot topic in physics, which might transmit data encoded in various spin patterns in future transistors, sensors or quantum computers.
The molecules used in the JILA experiments are made of one potassium atom bonded to one rubidium atom. The molecules are polar, with a positive electric charge at the rubidium end and a negative charge at the potassium end. This feature means the molecules can interact strongly and can be controlled with electric fields.
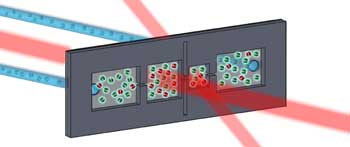
In the latest experiment, about 20,000 molecules were trapped in an optical lattice, an ordered pattern that looks like a stack of egg cartons created by intersecting laser beams. The lattice was only partly filled, with about one molecule per every 10 lattice wells. The lattice suppressed the molecules’ travel and chemical reactions, allowing their internal properties to guide interactions.
The JILA team used microwave pulses to manipulate the molecules’ spins, or natural rotations around an axis, similar to a spinning top, to create a “superposition” of two opposite spins at the same time. Scientists then observed oscillating patterns in the average spin of all the molecules, as well as a falloff or decay in the spin signal over time, indicating the molecules were swapping spins.
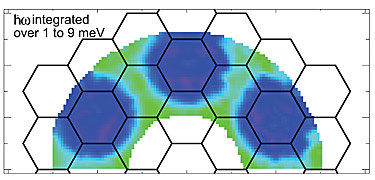
Scientists calculated the interaction energy that each molecule experiences with all other molecules in the lattice, with the energy intensity depending on the distance and angle between pairs (see graphic). JILA theorist Ana Maria Rey’s modeling of spin oscillations and time periods agreed with the experimental measurements. Ye says the spin-swapping interactions “entangle” the molecules, a signature feature of the quantum world that links the properties of physically separated particles.
The results are expected to open up a new field in which scientists create customized molecular spin models in solid-like structures held in place by the lattice. JILA scientists plan to fill the lattice more fully and add an external electric field to increase the variety of spin models that can be created.
The research was funded by NIST, the National Science Foundation, the Air Force Office of Scientific Research, the Army Research Office, the Department of Energy and the Defense Advanced Research Projects Agency.
*B. Yan, S.A. Moses, B. Gadway, J.P. Covey, K.R.A. Hazzard, A.M. Rey, D.S. Jin and J. Ye. Realizing a lattice spin model with polar molecules. Nature. Advance Online Publication, Sept. 18, 2013.
**See 2008 NIST news release, “JILA Scientists Create First Dense Gas of Ultracold ‘Polar’ Molecules,” at www.nist.gov/pml/div689/ultracold_polar_molecules.cfm; and 2010 NIST news release, “Seeing the Quantum in Chemistry” JILA Scientists Control Chemical Reactions of Ultracold Molecules,” at www.nist.gov/pml/div689/ultracold_021110.cfm.
Media Contact: Laura Ost, laura.ost@nist.gov, 303-497-4880