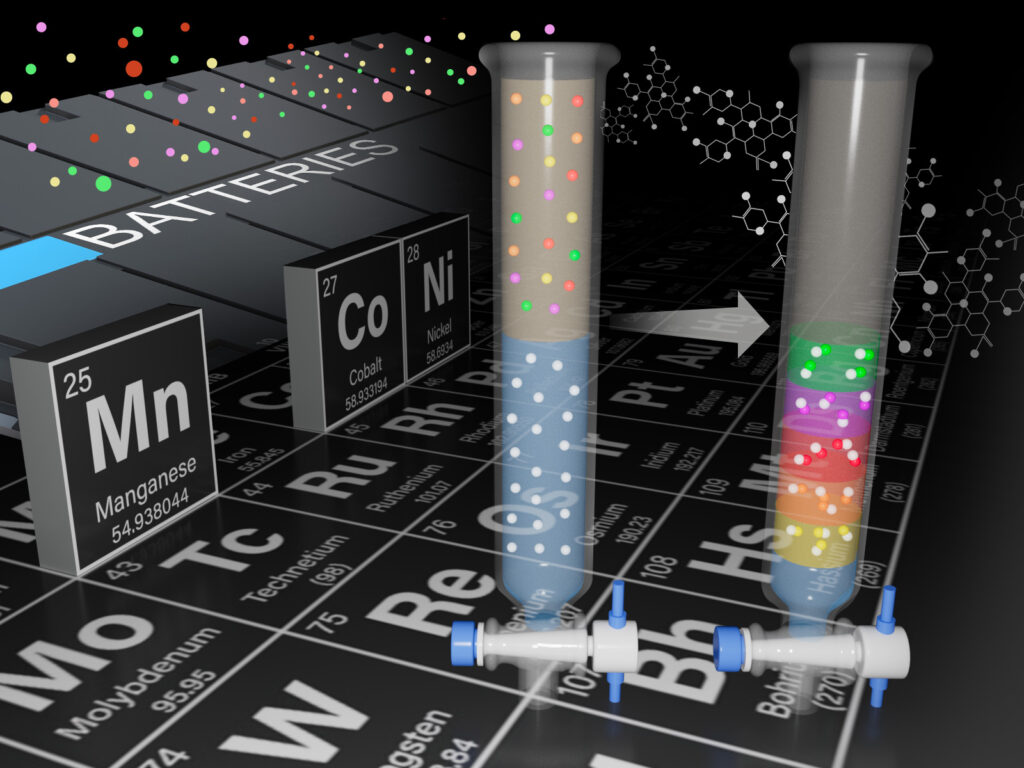
There’s some irony in the fact that devices that seem indispensable to modern life—mobile phones, personal computers, and anything battery-powered—depend entirely on minerals extracted from mining, one of the most ancient of human industries. Once their usefulness is spent, we typically return these objects to the Earth in landfills, by the millions.
But what if we could “mine” electronic waste (e-waste), recovering the useful minerals contained within them, instead of throwing them away? A clever method of recovering valuable minerals from e-waste, developed by a research team at the Department of Energy’s Pacific Northwest National Laboratory, is showing promise to do just that. Materials separation scientist Qingpu Wang presented recent success in selectively recovering manganese, magnesium, dysprosium, and neodymium, minerals critical to modern electronics, at the 2024 Materials Research Society’s Spring Meeting which took place in Seattle.
Go with the flow
Just as a prism splits white light into a dazzling rainbow of colors based on distinct wavelengths, so too can metals be separated from one another using their individual properties. However, current separation methods are slow, as well as chemical- and energy-intensive. These barriers make the recovery of valuable minerals from e-waste streams economically unfeasible.
In contrast, the PNNL research team used a simple mixed-salt water-based solution and their knowledge of metal properties to separate valuable minerals in continuously flowing reaction chambers.
The method, detailed in two complementary research articles and presented this week, is based on the behavior of different metals when placed in a chemical reaction chamber where two different liquids flow together continuously. The research team exploited the tendency of metals to form solids at different rates over time to separate and purify them.
“Our goal is to develop an environmentally friendly and scalable separation process to recover valuable minerals from e-waste,” said Wang. “Here we showed that we can spatially separate and recover nearly pure rare earth elements without complex, expensive reagents or time-consuming processes.”

The research team, which included materials scientist Chinmayee Subban, who also holds a joint appointment with the University of Washington, first reported in February 2024 successfully separating two essential rare earth elements, neodymium and dysprosium, from a mixed liquid. The two separate and purified solids formed in the reaction chamber in 4 hours, versus the 30 hours typically needed for conventional separation methods. These two critical minerals are used to manufacture permanent magnets found in computer hard drives and wind turbines, among other uses. Until now, separating these two elements with very similar properties has been challenging. The ability to economically recover them from e-waste could open up a new market and source of these key minerals.
Recovering minerals from e-waste is not the only application for this separation technique. The research team is exploring the recovery of magnesium from sea water as well as from mining waste and salt lake brines.
“Next, we are modifying the design of our reactor to recover a larger amount of product efficiently,” added Wang.
Recovering manganese from simulated battery waste
Using a complementary technique, Wang and his colleague Elias Nakouzi, a PNNL materials scientist, showed that they can recover nearly pure manganese (>96%) from a solution that mimics dissolved lithium-ion battery waste. Battery-grade manganese is produced by a handful of companies globally and is used primarily in the cathode, or negative pole of the battery.
In this study, the research team used a gel-based system to separate the materials based on the different transport and reactivity rates of the metals in the sample.
“The beauty in this process is its simplicity,” Nakouzi said. “Rather than relying on high-cost or specialty materials, we pared things back to thinking about the basics of ion behavior. And that’s where we found inspiration.”
The team is expanding the scope of the research and will be scaling up the process through a new PNNL initiative, Non-Equilibrium Transport Driven Separations (NETS), which is developing environmentally friendly new separations to provide a robust, domestic supply chain of critical minerals and rare earth elements.
“We expect this approach to be broadly relevant to chemical separations from complex feed streams and diverse chemistries—enabling more sustainable materials extraction and processing,” said Nakouzi.
The research studies reported at MRS received support from a Laboratory Directed Research and Development Program and the NETS initiative at PNNL.
Author Bio

Karyn Hede, Senior Science Communicator and Media Relations Advisor at PNNL
Karyn is an experienced science and medical communicator, author, and university instructor. She has worked both as a science journalist and as a media relations specialist. Karyn’s published work has appeared in Science, Nature, Scientific American, MIT’s Technology Review, Journal of the National Cancer Institute, and other media outlets. She has authored more than 70 published articles in the scientific and technical literature. She has also been an invited lecturer on science communication. She is passionate about making science and technology advances accessible to anyone curious about how scientists figure things out. At PNNL, she covers the physical and computational sciences, quantum information sciences, waste to fuel conversion, and vehicle technologies. Find out more about Karyn on LinkedIn.
About PNNL
Pacific Northwest National Laboratory draws on its distinguishing strengths in chemistry, Earth sciences, biology and data science to advance scientific knowledge and address challenges in sustainable energy and national security. Founded in 1965, PNNL is operated by Battelle for the Department of Energy’s Office of Science, which is the single largest supporter of basic research in the physical sciences in the United States. DOE’s Office of Science is working to address some of the most pressing challenges of our time. For more information, visit https://www.energy.gov/science/. For more information on PNNL, visit PNNL’s News Center. Follow us on Twitter, Facebook, LinkedIn and Instagram.